Kerala PSC Previous Years Question Paper & Answer
Page:4
Below are the scanned copy of Kerala Public Service Commission (KPSC) Question Paper with answer keys of Exam Name 'SCIENTIFIC OFFICER (CHEMISTRY) -KERALA POLICE SERVICE (FORENSIC SCIENCE LABORATORY) ' And exam conducted in the year 2021. And Question paper code was '020/2021'. Medium of question paper was in Malayalam or English . Booklet Alphacode was 'A'. Answer keys are given at the bottom, but we suggest you to try answering the questions yourself and compare the key along wih to check your performance. Because we would like you to do and practice by yourself.
10.
11.
12.
13.
14.
15.
Calculate the ionic strength I,, in a solution that contains 0.0200 mol KCl, 0.0050 mol
MgCl,, 0.0020 mol MgSO, and 100 g 70.
(A) 0.0430 mol.kg! (®) 0.0860 ایام
) 0.430 mol. kg"! (D) 0.860 17018
Read the following statements carefully :
(i) | The incident electron beam ionizes an atomic core level electron.
(ii) The energy released when a higher-energy electron falls back to fill the core level is
transferred to a valence electron, which is ejected into the gas phase where it is detected.
These processes involving in the principle of
(A) XPS (B) UPS
(C) Auger Electron Spectroscopy (D) LEED
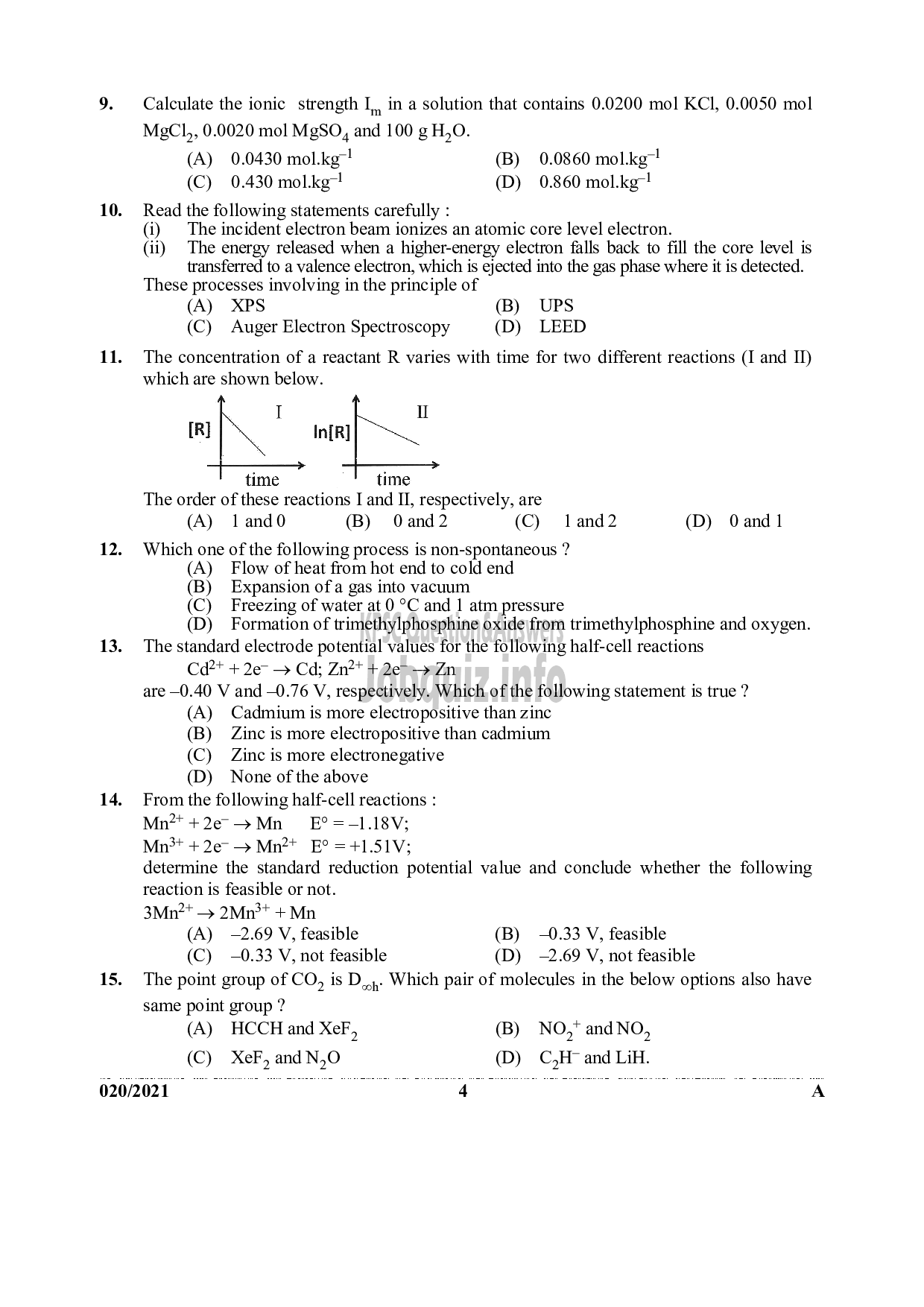
The concentration of a reactant R varies with time for two different reactions (I and 11)
which are shown below.
I (
IR] iN In{R]
പു >
time time
The order of these reactions I and II, respectively, are
(^) 18760 (8) 08162 (0 18102 (0) 1
Which one of the following process is non-spontaneous ?
(A) Flow of heat from hot end to cold end
(ம) Expansion of a gas into vacuum
(C) Freezing of water at 0 °C and 1 atm pressure
(D) Formation of trimethylphosphine oxide from trimethylphosphine and oxygen.
The standard electrode potential values for the following half-cell reactions
002+ +26 ೨. Cd; അ + 26 > Zn
are -0.40 V and -0.76 V, respectively. Which of the following statement is true ?
(A) Cadmium is more electropositive than zinc
(B) Zinc is more electropositive than cadmium
(C) Zinc is more electronegative
(D) None of the above
From the following half-cell reactions :
14112, + 26 -> षा = ८० =-1.18V;
1/13 +26 -> शा ° =+1.51V;
determine the standard reduction potential value and conclude whether the following
reaction is feasible or not.
3Mn?* > 21/03 + ماح
(A) -2.69 V, feasible ൫ -0.33 V, feasible
(6) -0.33 V, not feasible (D) -2.69 V, not feasible
The point group of CO, is D,,,. Which pair of molecules in the below options also have
same point group ?
(A) HCCH and XeF, (8) NO,* andNO,
(C) XeF, and N,O (D) C,H and LiH.
020/2021 : ` 4 ↽⊽ A